Why Partner in 2025?
Explore Related Events:
Introducing the Cell Therapies Event Series, a collection of 26 established events curated to enable this community to achieve their clinical and commercial potential.
Having trouble downloading the brochure? Let us know here and we'll email it to you instead.
An Exclusive Opportunity:
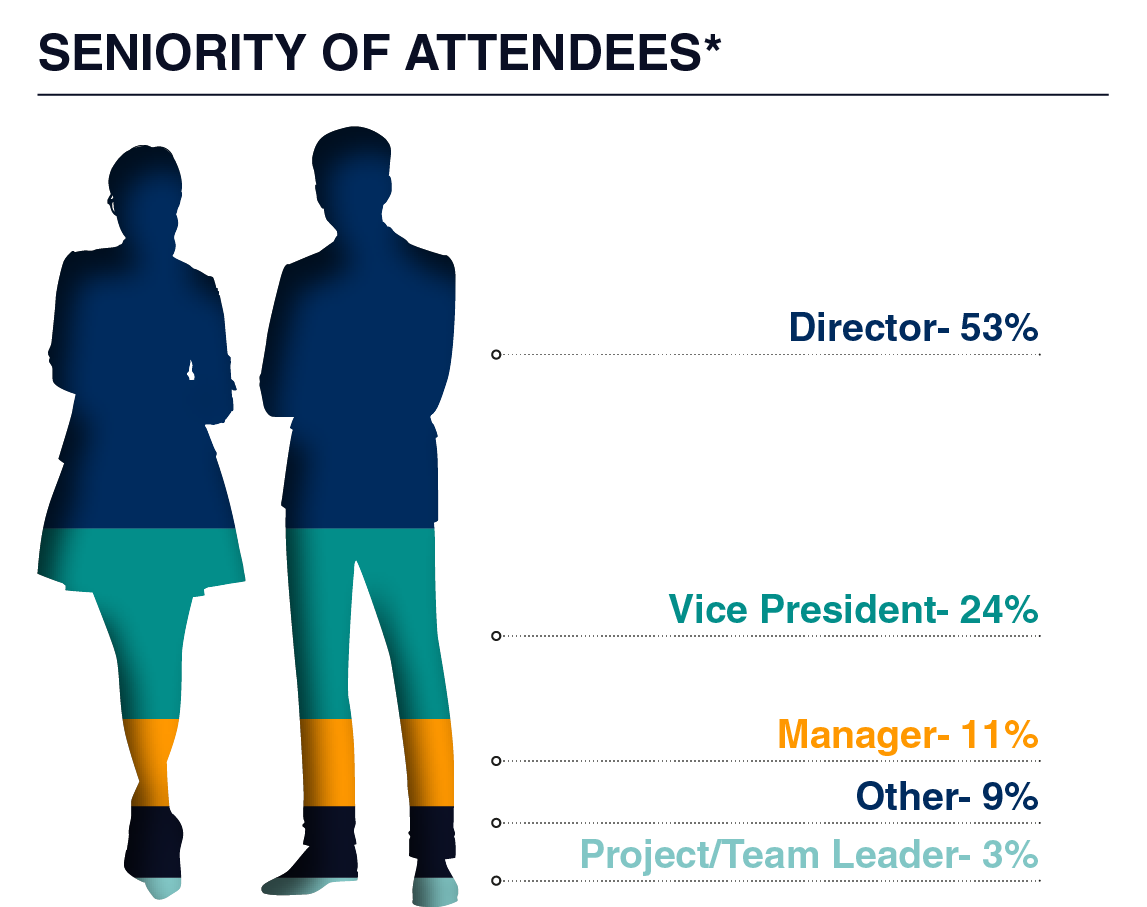
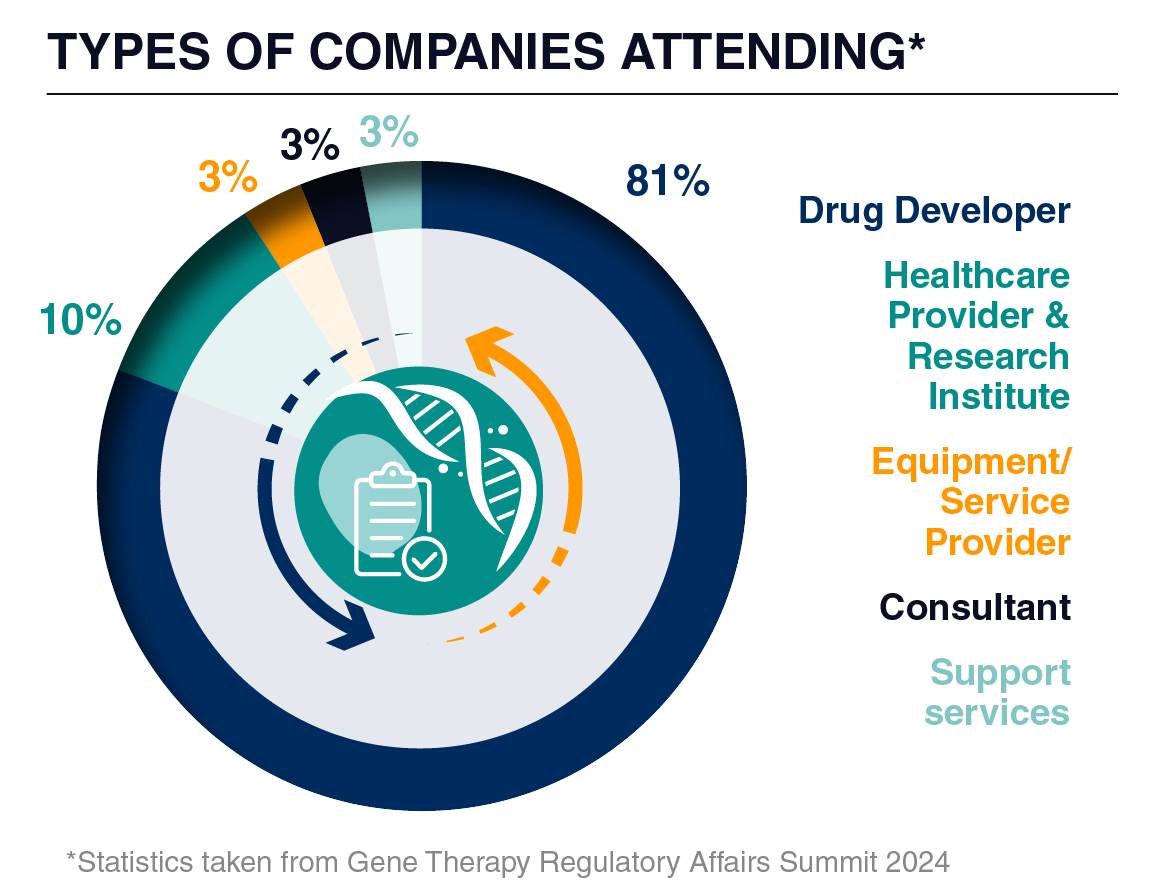
Our attendees seek consultancy firms, CROs, CDMOs, regulatory software providers, and more to propel their pipelines to the next stage of development. In 2025, you can meet C-Level Executive, Vice Presidents, and Directors of regulatory affairs and regulatory CMC, spanning from pre-IND to post-BLA, who seek your expertise to support filings, negotiations, understanding of current and new guidance, and much more!
As evolving guidance and new innovations challenge the traditional regulatory pathways, many cell and gene therapy developers are prioritizing investments in support to clarify frameworks and aid in negotiations with regulatory bodies.
Position yourself as the service provider of choice by showcasing your company at the 2025 Cell & Gene Therapy Regulatory Affairs Summit. This event promises a prime opportunity to network with regulatory leaders, decode biopharma needs, and build new relationships to exceed commercial goals.