Partner With Us
An Exclusive Opportunity:
Partner with the 3rd Cell & Gene Therapy Regulatory Affairs Summit to showcase your leadership in the fast-growing cell and gene therapy regulatory landscape. Unlike other events, this forum uniquely brings together global regulatory professionals, industry experts, and health authorities focused exclusively on CGT compliance and approval pathways.
By partnering, you gain direct access to decision-makers driving innovation, while positioning your brand at the forefront of critical regulatory updates, submission strategies, and best practices. This event offers unparalleled opportunities to build trust, influence policy discussions, and accelerate the development of transformative therapies.
Why Partner With the Summit?
Demonstrate Your Regulatory Expertise in Cell & Gene Therapy
Engage with Global Regulatory Professionals & Key Stakeholders
Access Critical Regulatory Updates & Best Practices
Position your company as a trusted leader in navigating complex regulatory frameworks and accelerating approvals in the rapidly evolving cell and gene therapy sector
Connect directly with regulatory affairs leaders, industry experts, and global health authorities shaping the future of cell and gene therapy compliance
Stay at the forefront of regulatory changes, submission strategies, and compliance challenges to enhance your offerings and support CGT development
Our Past Partners:


Experts Need Your Help With:
Seamless Regulatory Publishing & Medical Writing: Deliver polished, compliant submission documents that accelerate approvals and reduce back-and-forth with regulators
Cutting-Edge Regulatory Intelligence & Gap Analysis: Help clients stay ahead of shifting policies and craft winning global submission strategies
Strategic Consulting & Agency Engagement: Guide companies through complex regulatory pathways with expert insights and tailored support
Innovative Software Solutions: Empower regulatory teams with smart tools for efficient workflow, data accuracy, and global submission management
Seamless Regulatory Publishing & Medical Writing: Deliver polished, compliant submission documents that accelerate approvals and reduce back-and-forth with regulators
... And much more!
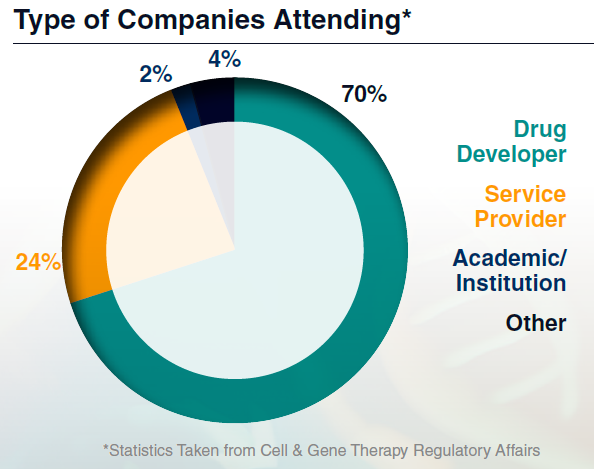
Who Will Be There?
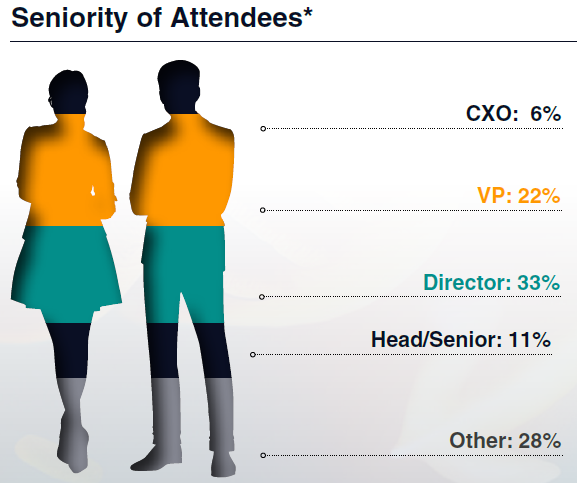
"Overwhelmingly positive things to say about the first Cell & Gene Regulatory Affairs Summit. I wanted to highlight its impactful discussions, cutting-edge insights, and collaborative spirit that all participants have enjoyed."
Previous Cell Therapy Series Sponsor, Director, Worldwide Clinical Trials
Explore Related Events:
Hanson Wade is committed to bringing the cell and gene therapy spaces bespoke conferences catering for every niche, with the aim of getting better drugs to patients faster.
Take a look at our related events below to find out more about our tailored conferences, designed to put you in the room with decision makers from both leading and emerging companies in these growing spaces.

Having trouble downloading? Let us know here and we'll email it to you instead.
"The talks are these meetings are from key industry leaders and very informative."
Previous Cell Therapy Series Sponsor, Senior Manager, Samsung Bioepis

