About Event
The Ultimate Chance to Tackle Regulatory Challenges in the Cell & Gene Therapy Space
In its second year, the Cell & Gene Therapy Regulatory Affairs Summit returned with a sharper focus and timely new content for 2025. As FDA and CBER leadership shifts reshaped expectations and AI began to influence submissions, regulatory professionals faced new complexities across IND, BLA, and global filings.
This was the only industry-led forum exclusively focused on CGT regulatory strategy, bringing together 18+ expert speakers, 3 global agencies, and more than 8 hours of networking. From evolving trial design to global alignment and AI-enabled tools, the agenda delivered essential insights to help accelerate compliant, high-quality approvals in a rapidly advancing field.
Discover Full Information Inside the 2025 Event Guide
- Learn how to manage regional misalignments and leverage expedited pathways
- Get practical guidance from real-world regulatory experiences
- Understand how to meet expectations while scaling complex therapies
5 Benefits Attendees Gained from This Year’s Summit:
Built Exclusively for CGT Regulatory Professionals
Agency & Industry Insights to Global Approvals
Aligning FDA, EMA & PMDA Requirements
Potency Assays, Comparability & Global Hurdles
In Vivo Gene Editing, AI & Advanced Platforms
Tailored for professionals managing nonclinical, clinical, and CMC submissions, it offers targeted insights to navigate complex, fast-evolving pathways
Hear directly from regulatory authorities like Health Canada and ANVISA as they share lessons learned from IND submission through to BLA and global market access
As CGT developers face divergent expectations from major regulators, this year’s sessions offer actionable strategies to align submissions across the FDA, EMA, PMDA, and other key agencies
Participate in pre-conference workshops tackling: establishing robust potency assays and demonstrating comparability, and navigating global submission roadblocks
Explore how regulators are responding to innovations like in vivo gene editing, AI-assisted regulatory workflows, and platform manufacturing strategies
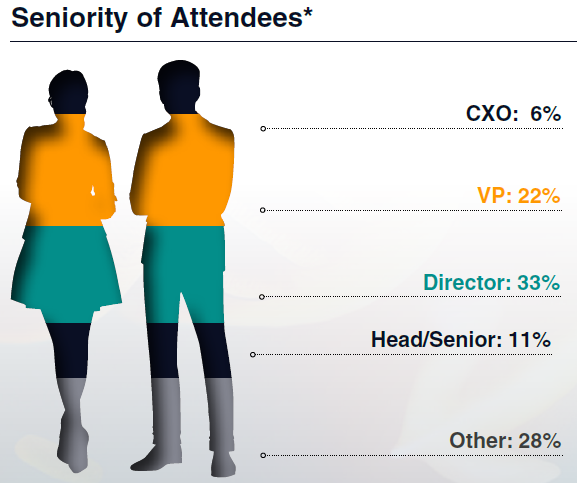
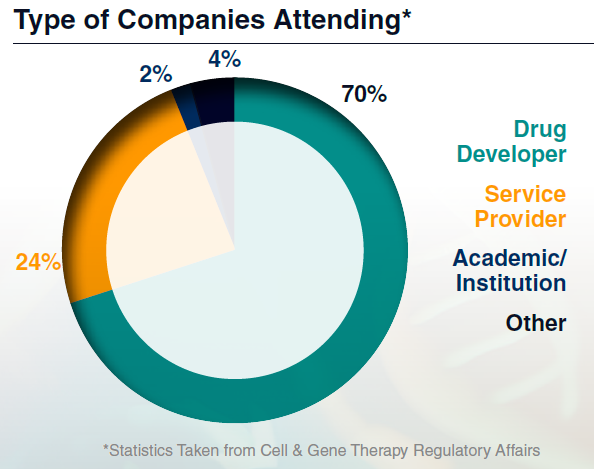
Who Did Attendees Meet?
Connect with a highly specialized audience of regulatory affairs professionals working across nonclinical, clinical, and CMC functions in cell and gene therapy. You'll meet regulatory leads from innovative biotechs, global pharma, regulatory consultancies and agencies who are actively navigating IND, BLA, and global submission pathways.
“All sessions were relevant and timely. The smaller attendee size allowed for interactive productive workshops and discussions/sharing of best practices during the conference based on current challenges and employed strategies by industry”.
2024 Attendee, Director Regulatory Intelligence, Regeneron
“The opportunity to reconnect with former colleagues and build new connections at this meeting was invaluable. I also appreciated hearing about the challenges faced by other companies and how they’re working to address them. It reminded me that we’re not operating in a bubble - many organizations are navigating similar issues and concerns.”
2024 Attendee, Director, Regulatory CMC, Umoja Biopharma
